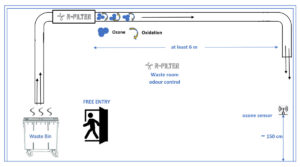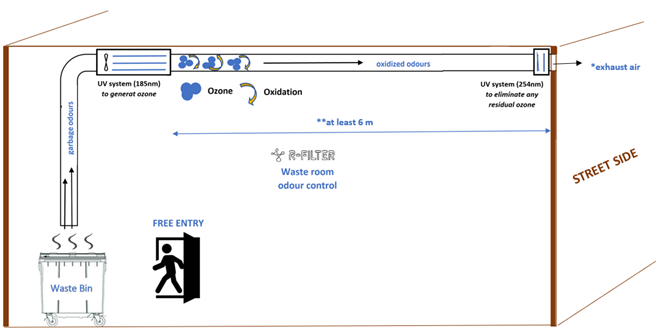
*exhaust air: If the exhaust air is discharged in a confined area or street level, it is essential that the ozone emission level measured at the outlet of the building does not exceed the local ozone safety levels for working areas.
**at least 6 m: Longer ducts allow ozone to remain in contact with odours for longer time to oxidize.
Why can UV radiation at 254 nm break down ozone (O₃)?
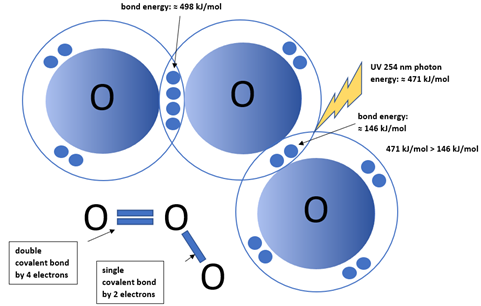
The bond energy of the O = O double bond is about 498 kJ/mol and the bond energy of the O - O single bond is approximately 146 kJ/mol. The energy of a UV photon of 254 nm is about 471 kJ/mol.
UV radiation at 254 nm can break down ozone because it provides enough energy to dissociate the ozone (O₃) molecule into oxygen (O₂) and an oxygen atom (O).