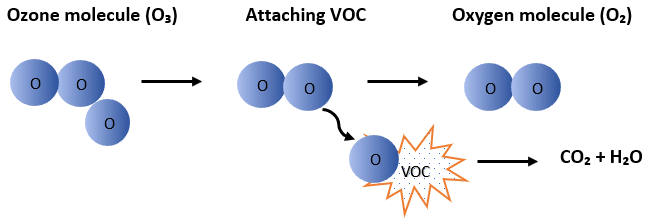
Ozone (O₃) is created naturally from oxygen (O₂), and it is also created in our devices with the help of 185 nm UV light. Through photolysis, nascent oxygen atoms are formed from the diatomic oxygen molecules in the air, which combine with the oxygen in the air to form ozone, which then strongly oxidizes the volatile organic compounds (VOCs), neutralizes unpleasant odors and disinfects the air.
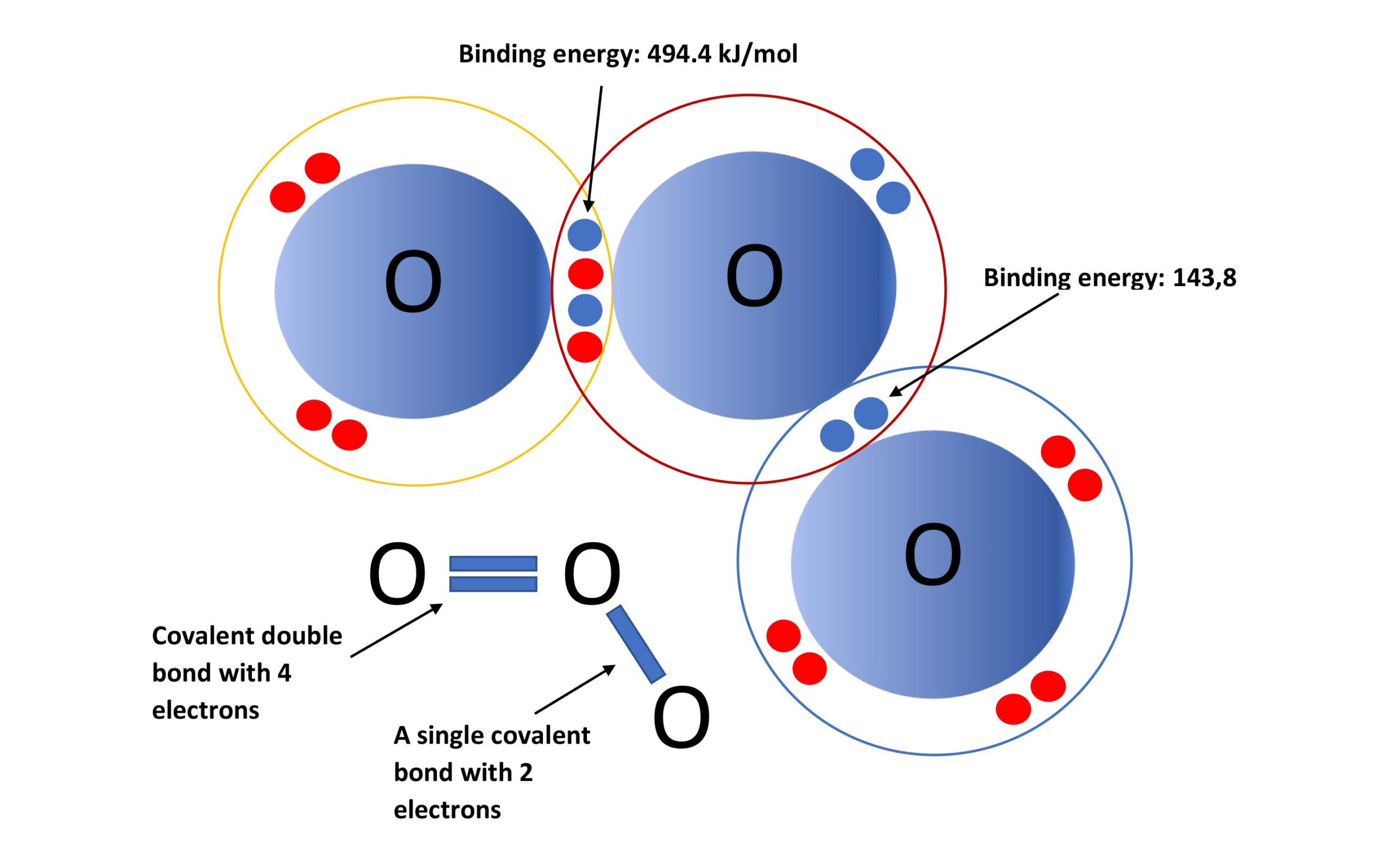
The figure above shows the electrons on the outer electron shell of each oxygen atom within the ozone molecule. It is the oxygen atom, with only a single covalent bond and lower bonding energy, that easily detaches from the ozone molecule and is used in the kitchen extractor duct to oxidise volatile organic compounds. Care must be taken to ensure that the ozone moves together with the air flow in the duct for at least 2-3 seconds so that the oxidising effect can occur.
Ozone is a suicide molecule. It searches for the VOC molecule to be attacked, oxidizes it, and destroys itself in the process.
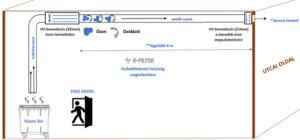
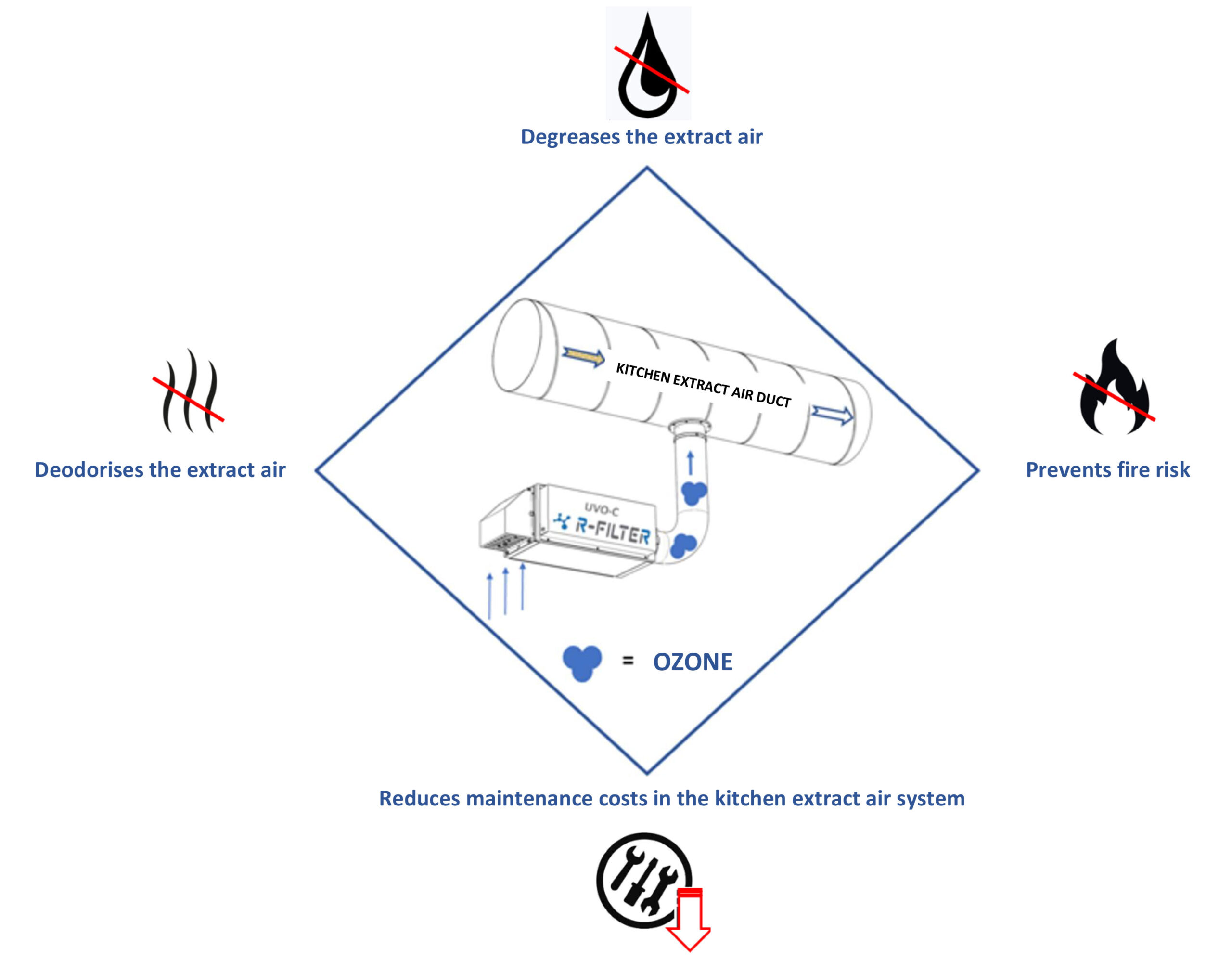
Our devices that are installed in the kitchen extract air duct:
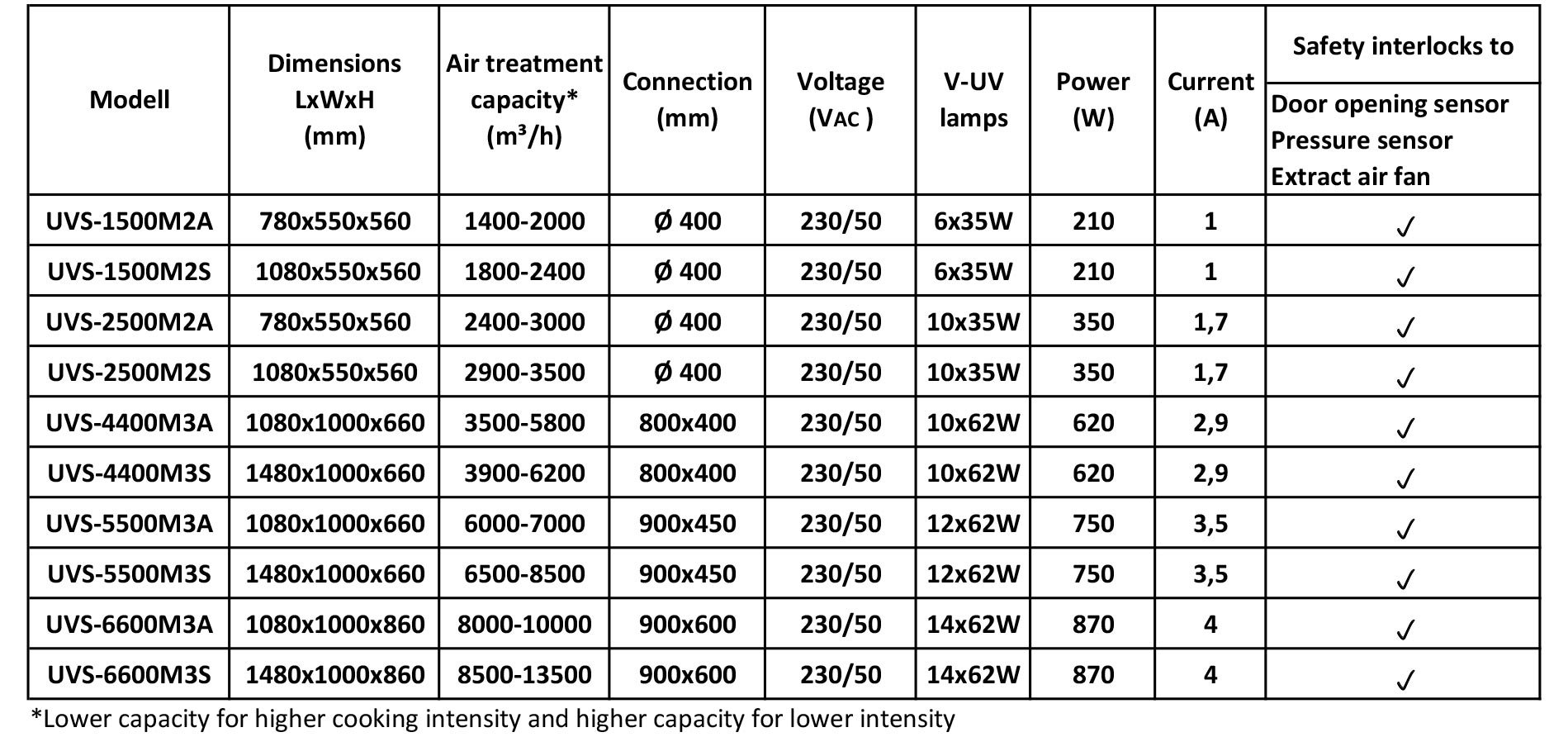
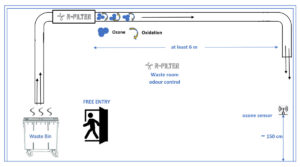
Our devices that inject ozone into the extract air duct from the outside: